Special Metal Spotlight: Nitinol
Nitinol, an alloy of nickel and titanium with approximately fifty percent nickel, is a relatively recently formulated metal that takes its name both from its composition and the place where it was produced (Nickel Titanium –Naval Ordnance Laboratory).
History of Nitinol
Although it was discovered in the early 1960s it took decades for Nitinol to become useful in the marketplace due to the difficulties encountered in its processing and manufacturing. In fact, Nitinol's many uses have mostly all been realized since the mid-1990s when its usefulness in a broad range of applications results derived from its shape memory characteristic and super-elastic properties. Shape memory means Nitinol can change to a new form at one temperature – lower than at normal body temperature for instance – and then recover its original shape upon being heated beyond its transformation temperature.

What are the characteristics and properties of Nitinol?
Since Nitinol also has a unique ability to adapt to extraordinary strains and is compatible with the human body it is finding numerous applications in the medical field. At higher temperatures, Nitinol assumes a cubic crystal structure referred to as austenite (also known as the parent phase). At lower temperatures, it spontaneously transforms to a more complicated ‘monoclinic’ crystal structure known as martensite. The temperature at which austenite transforms to martensite is generally referred to as the transformation temperature –more specifically, martensite begins to form at the so-called Ms temperature, and the temperature at which it is complete is called the Mf temperature. Those two facets of its structure – shape memory and superelastic properties – allow Nitinol to exhibit a reversible response to an applied stress which itself is caused by a phase transformation between the austenitic and martensitic phases of a crystal.
Crucial to Nitinol’s properties are two key aspects of this phase transformation. First is that the transformation is ‘reversible’, meaning that heating above the transformation temperature will revert the crystal structure to the simpler austenite phase. The second key point is that the transformation in both directions is instantaneous.
[1] Martensite's crystal structure has the unique ability to undergo limited deformation substantially without breaking atomic bonds. This type of deformation is known as twinning, which consists of the rearrangement of atomic planes without causing permanent deformation. It is able to undergo about 6–8% strain in this manner.
When martensite is reverted to austenite by heating, the original austenitic structure is returned, regardless of whether the martensite phase was deformed. Thus the name ‘shape memory’ refers to the fact that the shape of the high-temperature austenite phase is ‘remembered’, even though the alloy is severely deformed at a lower temperature.
[2] Thus Nitinol medical devices – such as stents – can be fabricated at body temperature, deformed or folded smaller at another temperature, then inserted into an artery where it will return to its normal temperature and regain is original size. Phase transformation also allows a device to fully recover after it has been bent to a high rate of strain (up to 7%).
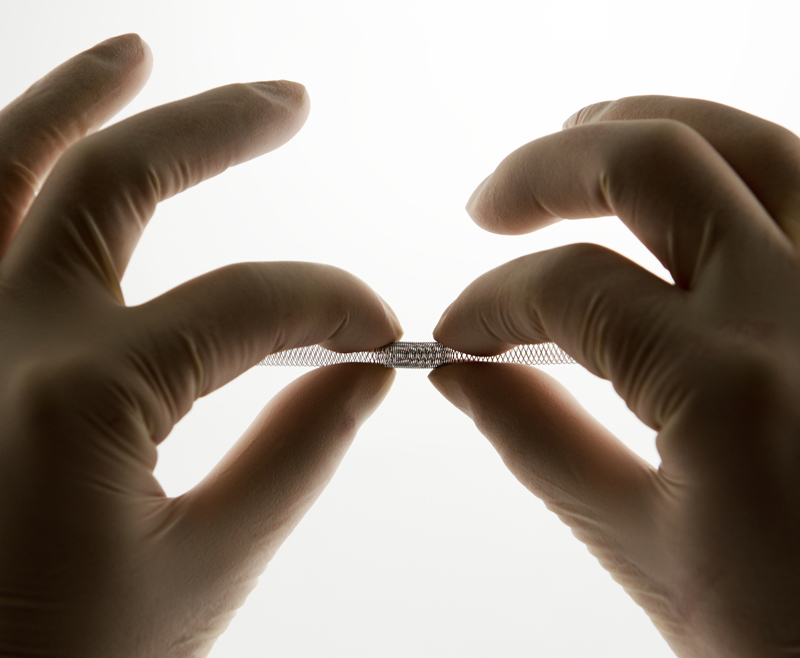
The super-elastic effect of Nitinol wire
This ‘superelastic’ effect allows use of nitinol wire devices that have been bent or shaped to allow introduction or use inside the body. Tools such as small grasping and biopsy devices can extend from a tube and expand to a much larger area than devices made from standard alloys. Nitinol's reduced weight and unique properties make it especially attractive for biomedical applications including heart valve tools, stents, staples, bone anchors, sophisticated septal defect devices and a variety of implants.
However, heat treating nitinol is delicate – and critical in fine tuning the transformation temperature. Aging time and temperature control the precipitation of various nickel-rich phases, and thus control how much nickel resides on the nickel–and titanium-lattice; by depleting the matrix of nickel, aging increases the transformation temperature. The combination of heat treatment and cold working is essential in controlling the properties of Nitinol
[3]. Nitinol's medical uses include devices for reconnecting intestines after surgery; as stitching; in implants; and wiring to locate breast tumors. Given its recent emergence in the manufacturing world, it is clear that the surface of Nitinol's future uses has barely been scratched.
Written by John Schmidt
References [1] http://www.nitinol.com/nitinol-university/nitinol-facts. [2] Funakubo, Hiroyasu (1984), Shape memory alloys, University of Tokyo, pp. 7, 176. [3] Pelton, A.; Russell, S. and DiCello, J. (2003), ‘The physical metallurgy of nitinol for medical applications’, JOM Journal of the Minerals, Metals and Materials Society, 55 (5): 33.



